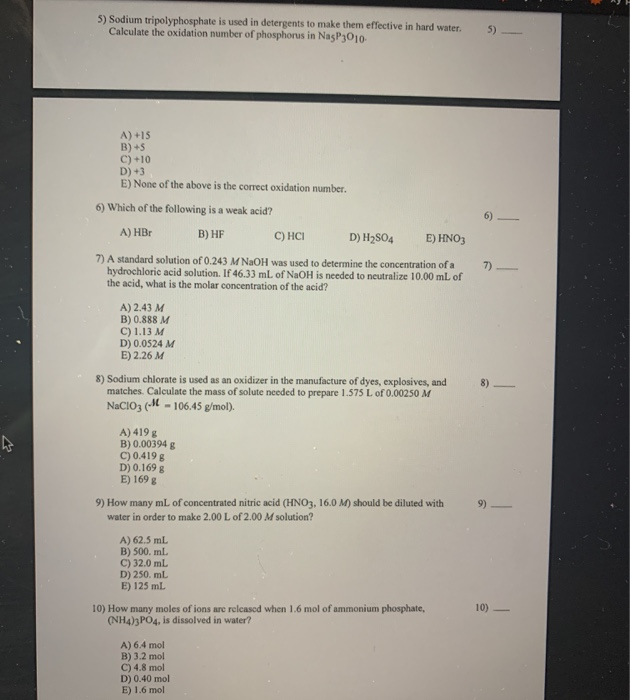
Calculate the Oxidation Number of Phosphorus in Na5p3o10
Sodium tripolyphosphate is used in bartleby. Phosphorus has a formal charge of _____ and an oxidation number of _____.

Answered 121 Sodium Tripolyphosphate Is Used In Bartleby
2 and 3 respectively.

. The oxidation number of group 1 elements is always 1. 6 Which of the following is a weak acid. The oxidation number of P in the compound PCl5 is 5.
That means the individual oxidation of each element adds up to zero. Calculate the oxidation number of the chlorine Cl in perchloric acid HClO4 a strong oxidizing agent. A 3 B 5 C 10 D 15 E None of the above is the correct oxidation number.
Calculate the oxidation number of sulfur in sodium metabisulfite Na2S2O5. The oxidation number of simple ions is equal to the charge on the ion. None of these is the correct oxidation number 32.
Sodium tripolyphosphate is used in detergents to make them effective in hard water. A 3 B 5 C 10 D 15 E None of the above is the correct oxidation number. Calculate the oxidation number of sulfur in sodium metabisulfite Na2S2O5.
Hydrogen has an oxidation number of 1 when combined with non-metals but it has an oxidation number of -1 when combined with metals. Calculate the oxidation number of phosphorus in Na5P3O10. Calculate the oxidation number of phosphorus in Na5P3O10.
A system that does no work but which receives heat from the surroundings has A q 0. The algebraic sum of the oxidation numbers of elements in a compound is zero. If 4633 mL.
Sodium hydroxide also known as caustic soda is used to neutralize acids and to treat cellulose in making of cellophane. The oxygens will always have a -2 charge and hydrogen is 1. Hence we have 3-2 coming from the 3 Oxygen atoms.
The II and III are the oxidation states of the iron in the two compounds. Calculate the oxidation number of phosphorus in NagP3010 5 7 A 15 B 5 C10 D 3 E None of the above is the correct oxidation number. Na in a compound is pretty much always 1.
5 P 1 Cl5. None of these is the correct oxidation number 32. Asked Sep 2 2019 in Chemistry by LittleBig.
Therefore the oxidation number of sulfur in Al2SO33 is 4. Sodium tripolyphosphate is used in detergents to make them effective in hard water. What is the oxidation number of iodine in I2.
Asked Sep 2 2019 in Chemistry by Physician. In which compound does phosphorus have an oxidation number of 3-. There is also a compound.
So the total positive charge from Hydrogen is 3 1 x 3 The total negative charge from Oxygen is -6 -2 x 3 The compound is electrically neutral so the phosphorus must have an oxidation state of 3. To find the correct oxidation state of P in H3PO4 Phosphoric acid and each element in the molecule we use a few rules and some simple mathFirst since t. Hence we have 31 coming from the 3 Sodiums.
That tells you that they contain Fe 2 and Fe 3 ions. Calculate the number of moles of HCl in 6285 mL of 0453 M hydrochloric acid. Have an oxidation number of 4 to balance the 6 of the oxygen atoms.
3H 1 x 3 3. The oxidation number of hydrogen in its compounds is 1 and the oxidation number of oxygen in its compounds is 2. This is one of the cases.
In Na3PO4 Na oxidation state is 1 and O oxidation state is -2. Sodium tripolyphosphate is used in detergents to make them effective in hard waten Calculate the oxidation number of phosphorus in NasP3O10. This can also be extended to the negative ion.
A HBr B HF C HCI D H2S04 E HNO3 7 A standard solution of 0243 M NaOH was used to determine the concentration of a hydrochloric acid solution. The compound is neutral meaning that the oxidation numbers will cancel each other out. The oxidation number of an atom is zero in a neutral substance that contains atoms of only one element.
The oxidation number of sodium in the Na ion is 1. Calculate the oxidation number of phosphorus in Na5P3O10. Thus the atoms in O 2 O 3 P 4 S 8 and aluminum metal all have an oxidation number of 0.
Calculate the oxidation number of phosphorus in Na5P3O10. As Na3PO4 is neutral so to do neutral -5 P shows 5 oxidation state. A 3 B 5 C 10 D 15 E none of the above is the correct oxidation number 2 See answers.
Oxidation number of H is 1 in most of the cases. 1P 3 x 1 3. Calculate the oxidation number of phosphorus in Na5P3O10.
Assign oxidation numbers to each atom and ion in the chemical equation. The phosphorus atom loses a total of 5 electrons one to each chlorine atom so its oxidation state will be 5. Assigning oxidation numbers to organic compounds.
Iron II sulphate is FeSO 4. Calculate the oxidation number of phosphorus in Na5P3O10. The oxidation number of a compound is 0.
Oxygen unless in an elemental or peroxide form will have an oxidation number of -2. That indicates oxidation number of the whole compound NaH2PO4 is 0. Asked Sep 3.
Sodium tripolyphosphate is used in detergents to make them effective in hard water. Assume that all the phosphorus atoms have the same oxidation number. Answer 1 of 2.
Calculate the oxidation number of phosphorus in Na5P3O10. The algebraic sum of the oxidation states in an ion is equal to the charge on the ion. So in Na3PO4 3-Na got 3 and 4-O got -8 charge which gives coverall -5 charge.

How To Find The Oxidation Number For P In H5p3o10 Tripolyphosphoric Acid Youtube

Solved Sodium Tripolyphosphate Is Used In Detergents To Make Chegg Com
Solved 5 Sodium Tripolyphosphate Is Used In Detergents To Chegg Com

How To Find The Oxidation Number For P In H5p3o10 Tripolyphosphoric Acid Youtube
Comments
Post a Comment